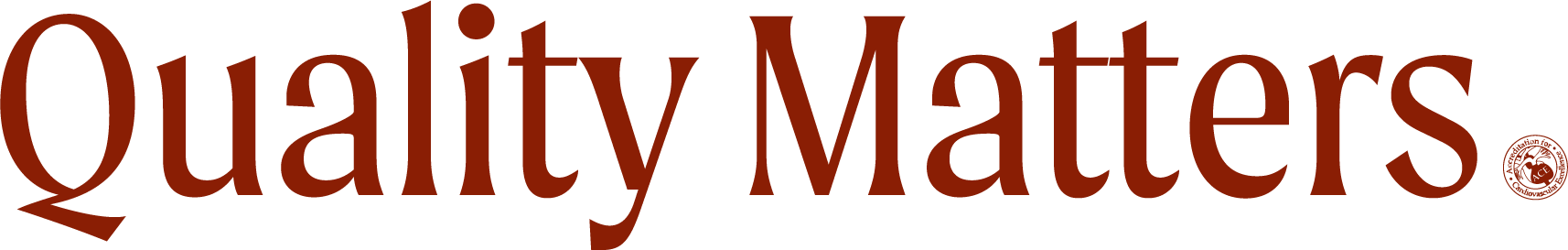Outcomes from trials focused on PCI device outcomes, drug-coated balloons, drug-eluting stents and bioresorbable stents caught our eye this week along with even more insight into the paclitaxel debate and the latest clinical perspectives coming out of the 2019 Vascular Interventional Advances (VIVA) annual meeting.

Nearly 1 Year Later, Endovascular Community Continues to Struggle With Paclitaxel – TCTMD
Discussions of clinical confusion surrounding paclitaxel-eluting devices were fruitful at VIVA 2019, offering practical insights into how to manage the clinical implications of uncertainty surrounding device and drug safety. Gary Ansel, MD of OhioHealth described how his large system called for an immediate moratorium on the devices while engaging a multi-disciplinary team to review the evidence. “I can tell you we went over the published literature in detail,” said Dr. Ansel. “It was refreshing for all of us to see the politics leave and just focus on the patients.” Read more »
Dr. Ansel’s insight here reinforces the value of multi-disciplinary teams focused on outcomes. Solid quality review and continuous quality improvement processes can not only improve patient care and provider excellence, but they can also help hospitals and systems be quick to act in cases of uncertainty like that of the paclitaxel debate. Accreditation for Cardiovascular Excellence can provide some often much-needed external impetus, support and validation for your organization to establish sustainable and robust quality improvement processes. Click here for more information on the ACE difference.
Boston Scientific Touts Positive Data on Two Paclitaxel Devices – Medical Device and Diagnostic Industry
While the paclitaxel debate may not be over, more positive data has come out since alarms were raised in December 2018, and Boston Scientific was at VIVA 2019 touting new clinical trial data showing positive safety and efficacy data for two paclitaxel products including the “Eluvia” stent which offers “sustained release of the lowest dose of paclitaxel of any peripheral drug-eluting device.” Read more »
Drug-coated Balloon Maintains Good Outcomes in 4-Year IN.PACT Global Study Data – Diagnostic and Interventional Cardiology
More cardiology device outcomes populated the late-breaking trials data at VIVA 2019. Four year outcomes from the IN.PACT global study seem to demonstrate long-term effectiveness of the use of drug-coated ballons for the treatment of femoropopliteal disease, with sustained clinical benefit and safety. Read more »
Bioresorbable Stent Shows Good 5-Year Outcomes in ABSORB BTK Trial – Diagnostic and Interventional Cardiology
Despite the Absorb bioresorbable drug-eluting stent being taken off the market due to poor sales — and even one study suggesting no additional benefit of Absorb over a conventional drug-eluting stent at three years — new five-year, single-center data shows a significant benefit for patients with peripheral artery disease. “These results represent best-in-class durability for a stent-like device in this challenging vascular territory,” said Ramon L. Varcoe, MBBS, MS, PhD. Read more »
LimFlow Announces Positive Six-Month Data From Promise I U.S. Early Feasibility Study – Cath Lab Digest
Interesting new technology—early feasibility data for the LimFlow system for the teatment of chronic limb-threatening ischemia is positive, with the novel approach showing an encouraging amputation-free survival rate of 74 percent and 100 percent of wounds classified as healed or healing at nine months follow-up. “The minimally-invasive LimFlow system is designed to bypass blocked arteries in the leg and delivery oxygenated blood back into the foot via the veins,” reads the press release. Read more »